Which of the Following Is True for a Heating Curve
Regions where the temperature increases as heat is added. 236 The time-temperature-transformation curve TTT curve for steel can best be described by which one of the following.

Heating Curve For Water Introduction To Chemistry
It shows how the pressure of a substance changes when heated.
. We review their content and use your feedback to keep the quality high. OD It shows how the mass of a substance changes when heated. The gas is a metastable state.
Select the correct answer. A indicates how annealing is used to achieve a given desired microstructure b indicates how cooling rate affects the transformation of austenite into various possible phases c indicates how martensite is gradually transformed into tempered. The substance supercools easily.
While anywhere along the line segment BD represents a phase change from solid to liquid and points Y and Z are both on that line the correct answer is D. The heat capacity of the gas is greater than that of the liquid. Assign the appropriate phases on the heating curve shown below.
Experts are tested by Chegg as specialists in their subject area. It shows how the volume of a substance changes when heated. The final temperature of the vapor is 1400C.
It shows for the volume of a substance changes when heated. They leave the surroundings feeling cold. Another way to model water is with letters and lines is like this.
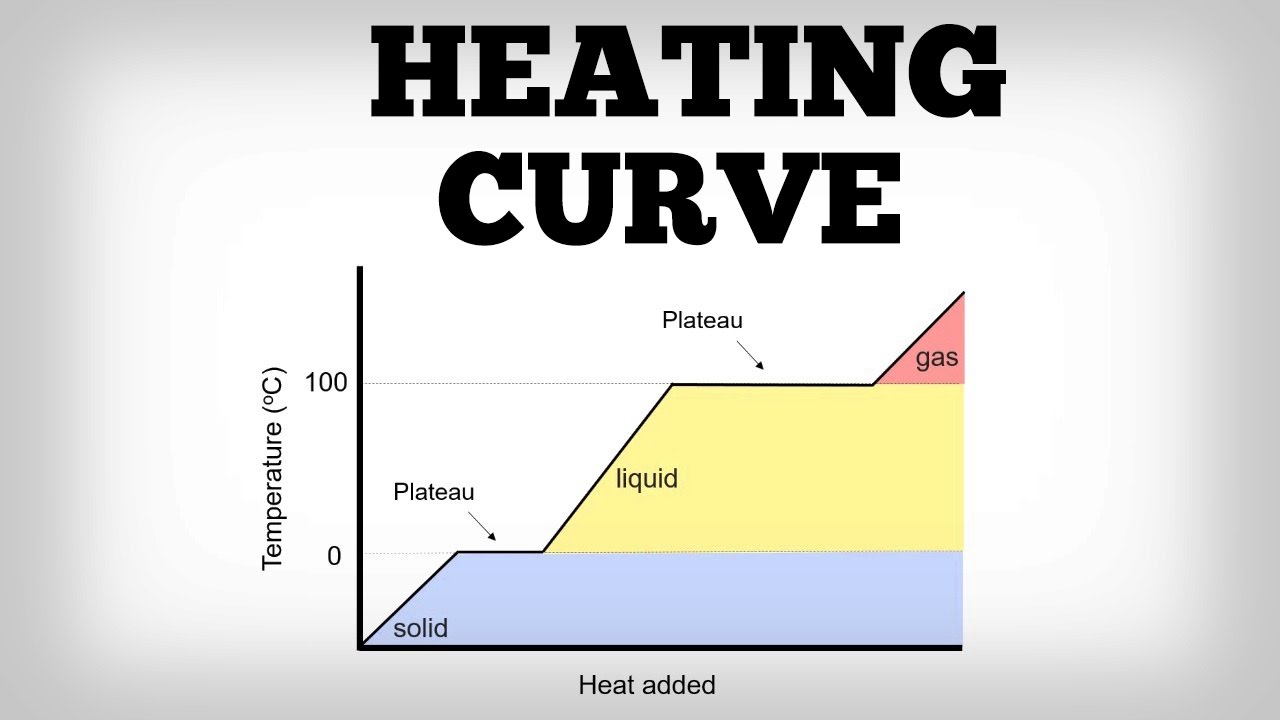
Heating Curve Worksheet PART A. It shows how the temperature of a substance changes when heated. Which of the following correctly describes a heating-cooling curve.
The melting and boiling points of the substance can be determined by the horizontal lines or plateaus on the curve. A plot of heat vs. What a substance looks like as it is heated b.
It shows how the mass of a substance changes when heated. The heating curve for a sample of pure ethanol is provided above. Which of the following is true for a heating curve.
The chemical changes that occur as the substance is heated. What a substance looks like as it is heated. The changes in the temperature and physical state of a substance as it is heated.
Which of the following statements are true about heating curves. What happens to the particles of a substance as it is heated. 1 Adenine 2 Cytosine 3 Guanine 4 Thymine 5 Uracil.
It shows how the temperature of a substance changes when heated. The temperature was recorded as a 500 g sample of solid ethanol was heated at a constant rate. They release energy into the surroundings.
There are two main observations on the measured curve. Which of the following is true for a heating curve. The change of state behavior of all substances can be represented with a heating curve of this type.
Which of the following statements are true about heating curves. What happens to the heat applied as the temperature is increased. A heating cooling curve shows the changes that occurs when ____ is added to or removed from a sample of matter at a _____ rate Heat constant Within a phase a change in heat causes the temperature of the substance to _____ as the _____ energy of the molecules changes.
Ice is melting to form liquid water. Choose as many as apply. Liquid water is becoming warmer.
Which of these conditions is always true for an exothermic process. Which of the following explains why the slope of segment T is greater than the slope of segment R. The heat capacity of the gas is greater than the heat of fusion.
A constant rate of heating is assumed so that one can also think of the x-axis as the amount of time that goes by as a substance is heated. Temperature is plotted on the y-axis while the x-axis represents the heat that has been added. What happens to the particles of a substance as it is heated.
A heating curve illustrates what a substance looks like as it is heated. The following graph is a heating curve showing the addition heat at a constant rate of 5000 joulesminute to a 300 gram sample of ice of at 200C. The substance must be a salt that dissociates on heating.
On the basis of this heating curve which of the following statement is correct about the substance. Other substances would of course have melting and boiling points that are different from those of water. A heating-cooling curve is constructed by adding or removing heat at a constant rate.
Choose as many as apply Which nitrogenous base is NOT found in RNA. What happens to the heat applied as the temperature is increased. The changes in the temperature and physical state of a substance as it is heated.
It shows how the pressure of a substance changes when heated. On any heating curve energy is added and therefore it is always endothermic. A cooling curve is the reverse of a heating curve - the only difference is the sign assigned to the calculated value.
A heating curve and phase diagram for water. What is one way that these. What is occurring during the portion of the heating curve labeled C.
A heating curve illustrates a. Temperature for a substance that is heated or cooled at a constant rate and constant pressure Reason. On any cooling curve energy is released and therefore it is always exothermic.
At point Y the phase change is occurring at the same pressure 1 atm that was used to construct the heating curve.

If The Heating Curve Is Reversed What Describes The Boiling Point Lisbdnet Com

Heating And Cooling Curves Also Called Temperature Curves Chemistry For Non Majors

No comments for "Which of the Following Is True for a Heating Curve"
Post a Comment